PBS is commonly used in biochemistry. Phosphoric acid or sodium hydroxide NaOH 4.
138 in sufficient H 2 O to make a final volume of 1 L and dissolve 142 g of Na 2 HPO 4 dibasic.
. Double-check that youre using exactly 1 L 42 c for your buffer. Add dH2O until the volume is 1 L. Best results for sodium phosphate buffer ph calculation-37.
02 M monobasic stock. Intas Pharmaceuticals Ltd. To prepare the sodium phosphate buffer of 003 M pH 70.
02M dibasic sodium phosphate 1 liter Na2HPO412H2O MW35814 -----7164gm dH2O to make 1 liter Solution X 02M. Prepare 800 mL of dH2O in a suitable container. Change the value in the textbox above to scale the recipe volume Table 1.
3 Measure the pH of the solution. To prepare a 08 M phosphate buffer stock combine the above ingredients in H 2 O. Principle And Interpretation Phosphate buffer pH 800 is formulated as described in USP 1.
Phosphate Buffer pH 80 002 M. Add 20214 g of Sodium Phosphate Dibasic Heptahydrate to the solution. Buffer solution pH 30.
500 mL dH 2 O. If it is above the desired pH add phosphoric acid to lower it to the desired. To make your phosphate buffer youll need the following materials.
Type the desired pH into the first cell and type the intended buffer strength in millimoles per liter in the second cell. Phosphate Buffer Ph Table - 17 images - preparation of sodium phosphate buffers image result for prepare phosphate buffer solution ph 6 8 chemistry general chemistry acid base titration and buffers ii reagents used to prepare the phosphate buffers download table. Then I add in 1 Triton X-100 into my.
Prepare 01 M sodium phosphate dibasic. Dissolve 78 g ofsodium dihydrogen phosphate Rin 900 mL of water RadjusttopH28withphosphoric acid R and dilute to 1000 mL with the same solvent. 02 M dibasic stock.
Put 80 mL of sodium phosphate dibasic stock 05 M from Step 1 in a beaker and add H2O to give a final volume of. 1 In a beaker pipette aliquots of 1M stock solutions according to the desired pH of your buffer see table below. Base 0523 molesL.
The ratio refers to the acid-base conjugate pair that corresponds to that pKa. Phosphate buffer solution pH 28. Mix 50 ml of 02 M potassium dihydrogen phosphate with 468 ml of 02 M sodium hydroxide and add sufficient water to produce 500 ml.
If it is below the desired pH add NaOH to raise it to the correct pH. Phosphate buffer is a multi-purpose buffer used for many molecular biology procedures. 156 g sodium phosphate monobasic dihydrate NaH 2 PO 4 2H 2 O MW 15599 gmol or 139 g of the anhydrous form.
For a 1 M buffer selected to make the calculation easy Acid Base 1. First calculate 50mM KCl for your desired volume and dissolve it in K 2 HPO 4 stock buffer and adjust with KH 2 PO 4 stock buffer as attached table and make up the final. Dissolve 210 g ofcitric acid R in 200 mL of 1Msodium hydroxide and dilute to 1000 mL with water R.
Base 062acid equation 1 You want 01M x 1 L 01 mol and. To prepare the stock solutions dissolve 138 g of NaH 2 PO 4 H 2 O monobasic. Phosphate Buffered Saline PBS 1 L 09 wv sodium chloride in 10 mM phosphate buffer pH 74 NaCl 800 g KCl 020 g Na 2HPO 4 144 g KH 2PO 4 024 g diH Phosphate-buffered saline abbreviated PBS is a buffer solution pH 74 commonly used in biological researchIt is a water-based salt solution containing disodium hydrogen phosphate.
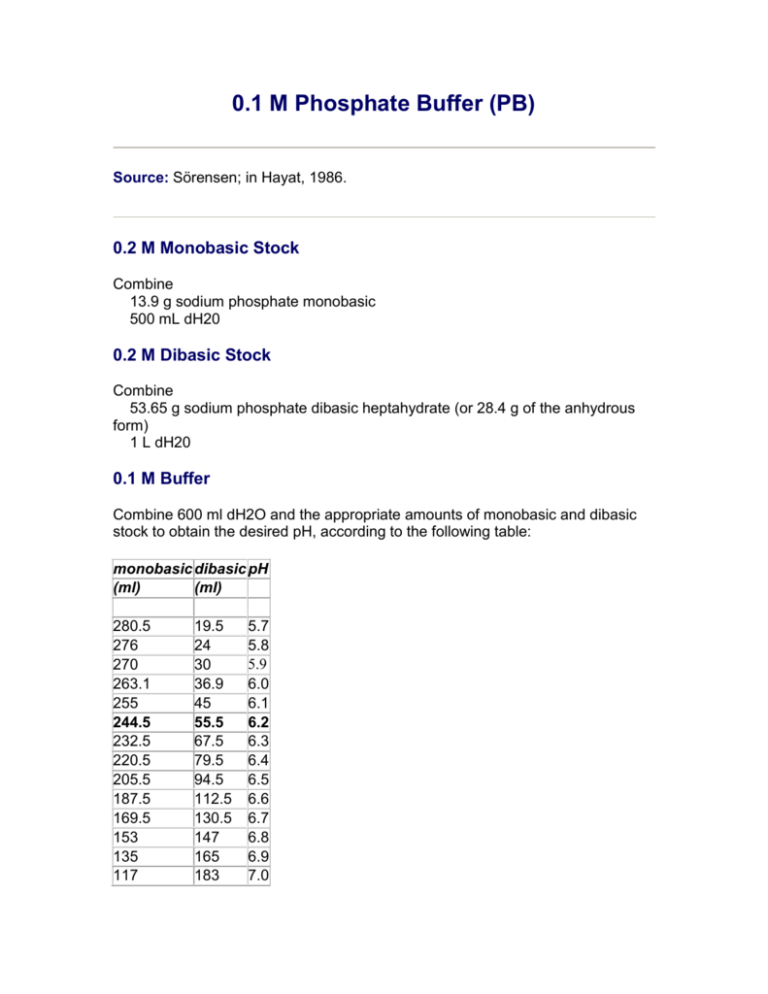
Mixing 1 M NaH 2 PO 4 monobasic and 1 M Na 2 HPO 4 dibasic stock solutions in the volumes designated in the table below results in 1 L of 1 M sodium phosphate buffer of the desired pH. PH meter and probe 5. Add 16282 g of Potassium phosphate dibasic to the solution.
Base 1 - Acid. Step 1 Pour 2 L 85 c of distilled water into 2 large reusable containers. Use the pKa value nearest your desired pH.
Substitute this into the ratio and solve. 2 Add water to bring the volume to approximately 45 mL. The molarity of the buffer is the sum of the molarities of the acid and conjugate base or the sum of Acid Base.
Phosphate buffer pH 68 preparation protocol below Stock solutions. 01 M sodium phosphate buffer pH 74 Add 31 g of NaH 2 PO 4 H 2 O and 109 g of Na 2 HPO 4 anhydrous to distilled H 2 O to make a volume of 1 L. Thanks for the information.
To prepare L of Potassium Phosphate pH 58 to 80. Heat if necessary to dissolve the medium completely. Press the calculate button and the approximate concentrations of monosodium phosphate monohydrate and disodium phosphate heptahydrate will be displayed.
Sterilize by autoclaving at 121C for 15 minutes. Phosphate Buffer pH 58 to 74 Preparation and Recipe. Each container should have 1 L 42 c of distilled.
How to make phosphate buffer. 385 mL of Na 2 HPO 4. April 20th 2018 - citric acid sodium phosphate buffer ph range 2 6 7 6 prepare a 0 1m solution of citric acid monohydrate c 6h8 o 7 h 2o 21 01g l and acalculations for a buffer soluti interchemnet home april 26th 2018 - calculations for a buffer solution of a given ph and buffering capacity sodium phosphate this means that 100 ml of.
Add 8878 mg of Potassium Phosphate Monobasic to the solution. 150 ml of 01 M sodium phosphate buffer pH 80 was taken and pH was adjusted to 70 and volume made. To prepare the 01 M sodium phosphate buffer 02 M of NaOH was added to 250 ml of 002 M NaH 2 PO 4 2H 2 O to adjust pH to 80 and volume made up to 500 ml with distilled water DW.
Ratio of Base Acid 1096. Monobasic potassium phosphate 0523 Final pH at 25C 80 Formula adjusted standardized to suit performance parameters Directions Suspend 1725 grams in 1000 ml distilled water. Phosphate Buffer 0025 M Standard.
So if I need to prepare lysis buffer 01M sodium phosphate buffer containing 1 Triton X-100 at pH 68 I prepare and adjust a high concentration stock of phosphate buffer making final adjustments with the mono or dibasic stock until I get pH 68 then dilute to the working buffer concentration. Dissolve 340 g of potassium dihydrogen phosphate and 355 g of anhydrous disodium hydrogen phosphate both previously dried at 110 to 130 for 2 hours in sufficient. Add 3394 g of Sodium Phosphate Monobasic Monohydrate to the solution.
Prepare 800 mL of distilled water in a suitable container. Adjust solution to final desired pH using. Always use distilled water instead of tap so the pH can be as accurate as possible3 X Research source You can find distilled water at most stores selling laboratory supplies.
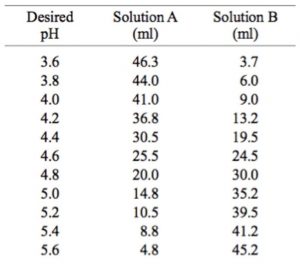
Buffer Formulations Exalpha Biologicals Inc

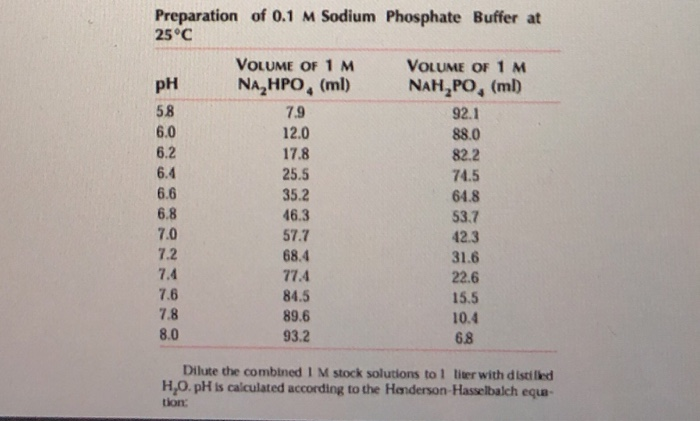

0 Comments